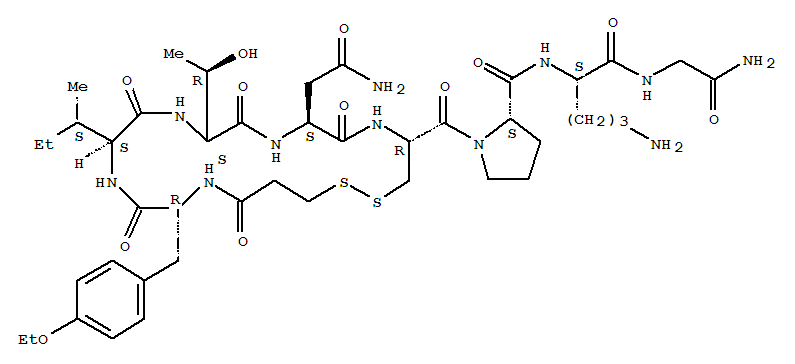
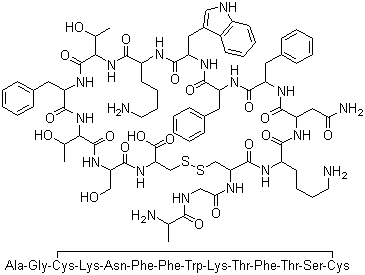
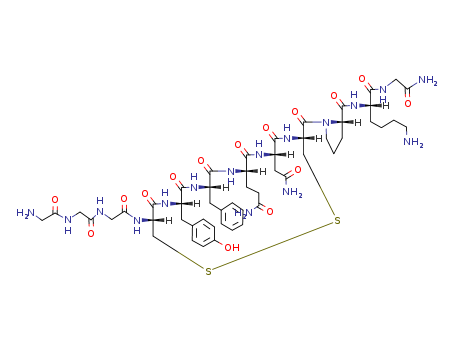
The raw material of cetuximab acetate has passed the GMP compliance inspection
时间:2024.12.04
On Jun. 12th 2024, the Heilongjiang Provincial Drug Administration announced the results of the GMP compliance inspection of Jixianglong "Cetrorelix Acetate" API. The results showed that after on-site inspection, it met the requirements of the "Good Manufacturing Practice for Pharmaceuticals (Revised in 2010)".
Jixianglong company accepted the drug GMP compliance inspection of the Heilongjiang Provincial Drug Administration from May 9 to 11, 2024, and the inspection scope was the API (Cetrorelix Acetate). After passing this compliance inspection, it became the company's ninth peptides API variety to pass the GMP compliance inspection after Atosiban acetate, Terlipressin acetate, Oxytocin, Octreotide acetate, Somatostatin, Eptifibatide, Thymofasin, and Carbetocin. This variety has been approved for marketing application of chemical APIs in March 2023.
Cetrorelix is a synthetic peptide, and its indication is to prevent premature ovulation in patients undergoing controlled ovarian stimulation, and then perform egg collection and assisted reproductive technology treatment. This product was first launched in Germany in 1999, in the United States in 2000, and in China in 2010. The advantages of Cetrorelix are that it has a short use time, few adverse reactions, and can achieve the same therapeutic effect as GnRHa.